20.6.1. Review: Preparation of enolates
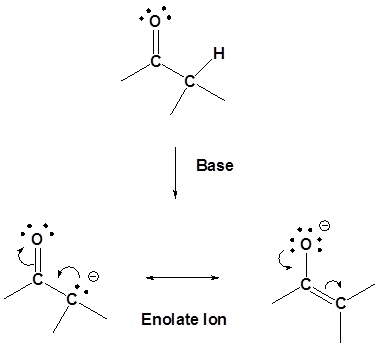
The enolate has two resonance forms – the negative charge can be either on carbon or oxygen – but enolates usually react as nucleophiles from the carbon, as we saw in section 9.7. with enolate alkylation SN2 reactions.
When we first studied enolate chemistry previously in section 9.7, we used LDA to generate enolates which then reacted as nucleophiles in SN2 reactions. For these alkylation reactions to be useful, the enolate anions must be generated in high concentration in the absence of other strong nucleophiles and bases. Aqueous base (e.g., aq. NaOH) and alkoxides (e.g., NaOCH2CH3) are usually not be suitable because they produce only low concentrations of the enolate anions, and the remaining -OH or -OR can cause unwanted side reactions. In other words, these nucleophilic bases will simply react directly with the alkyl halide via an SN2 reaction.




If the formed enolate is stabilized by more than one carbonyl it is possible to use a weaker base such as sodium ethoxide to form the enolate almost quantitatively.
NaOCH2CH3 = Na+ –OCH2CH3 = NaOEt

Enolates are very useful in synthesis, as they represent a stabilized nucleophilic form of carbon. This chart shows the range of reactions that can be used:

We will examine the aldol reaction next. The Claisen condensation will be covered later, in section 22.2.
20.6.2. Basic aldol reaction
General Aldol reaction
If a base is added a low temperatures to an aldehyde or ketone that has an alpha hydrogen, an enolate is formed which immediately undergoes a nucleophilic addition across the C=O of another molecule of aldehyde or ketone. As long as the reaction is kept cold, a beta-hydroxyaldehyde (often called an “aldol”) or a beta-hydroxyketone product can be isolated. Since the only electrophile present is the aldehyde/ketone, a weaker base such as NaOH or NaOCH3 can be used.

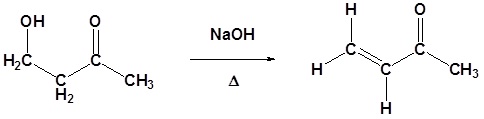
If the reaction is warmed, it can then lose a molecule of water to form an alkene-aldehyde or alkene-ketone, known as an alpha,beta-unsaturated aldehyde or ketone. In this case the overall reaction is known as an aldol condensation.
Aldol condensation

Aldol condensations are important in organic synthesis, because they provide a good way to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or “aldol” (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals.

The name aldol condensation is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, the aldol reaction is not formally a condensation reaction because it does not involve the loss of a small molecule.
The reaction between an aldehyde/ketone and an aromatic carbonyl compound lacking an alpha-hydrogen (cross aldol condensation) is called the Claisen-Schmidt condensation. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this topic in 1880 and 1881. An example is the synthesis of dibenzylideneacetone. Quantitative yields in Claisen-Schmidt reactions have been reported in the absence of solvent using sodium hydroxide as the base and plus benzaldehydes.
Mechanism
The first part of this reaction is an aldol reaction, the second part a dehydration—an elimination reaction (Involves removal of a water molecule or an alcohol molecule). Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present. The aldol addition product can be dehydrated via two mechanisms; a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride in an enolate mechanism, or in an acid-catalyzed enol mechanism. We will focus on the base-catalyzed mechanism, which is more widely used.
Enolate mechanism
If the catalyst is a moderate base such as hydroxide ion or an alkoxide, the aldol reaction occurs via nucleophilic attack by the resonance-stabilized enolate on the carbonyl group of another molecule. The product is the alkoxide salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.
Base-catalyzed aldol reaction (shown using −OCH3 as base)
Base-catalyzed dehydration
Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a stoichiometric amount of a strong base such as LDA. In this case, enolate formation is irreversible, and the aldol product is not formed until the alkoxide of the aldol product is protonated in a separate acid-base workup step. Mixtures of stereoisomers (E & Z) are obtained from some reactions, though the E product is generally favored. Overall the general reaction involves a dehydration of an aldol product to form an alkene:
Going from reactants to products simply

Figure: The aldol condensation example
Example 2: Aldol Condensation
Intramolecular aldol reaction
Molecules which contain two carbonyl functionalities have the possibility of forming a ring through an intramolecular aldol reaction. This is where the “head” of the molecule “bites its own tail”. In most cases two sets of $$\alpha$$ hydrogens need to be considered. As with most ring forming reaction five and six membered rings are preferred.

As with other aldol reactions, the addition of heat causes an aldol condensation to occur.

Mixed aldol reaction and condensations
The previous examples of aldol reactions and condensations used a common reactant as both the enolic donor and the electrophilic acceptor. The product in such cases is always a dimer of the reactant carbonyl compound. Aldol condensations between different carbonyl reactants are called crossed or mixed reactions, and under certain conditions such crossed aldol condensations can be effective.
Example 4: Mixed Aldol Reactions

The success of these mixed aldol reactions is due to two factors. First, aldehydes are more reactive acceptor electrophiles than ketones, and formaldehyde is more reactive than other aldehydes. Second, aldehydes lacking alpha-hydrogens can only function as acceptor reactants, and this reduces the number of possible products by half. Mixed aldols in which both reactants can serve as donors and acceptors generally give complex mixtures of both dimeric (homo) aldols and crossed aldols. Because of this most mixed aldol reactions are usually not performed unless one reactant has no alpha hydrogens.
The following abbreviated formulas illustrate the possible products in such a case, red letters representing the acceptor component and blue the donor. If all the reactions occurred at the same rate, equal quantities of the four products would be obtained. Separation and purification of the components of such a mixture would be difficult.
AACH2CHO + BCH2CHO + NaOH → A–A + B–B + A–B + B–A
The aldol condensation of ketones with aryl aldehydes to form α,β-unsaturated derivatives is called the Claisen-Schmidt reaction. This relies on the fact that the addition of an enolate to a ketone is not particularly favorable, whereas the addition of an enolate to an aldehyde is favorable; this means that the aldehyde is consumed, while the ketone serves to provide the enolate part for the reaction.
Example 4: Claisen-Schmidt Reaction

Another approach is to use LDA on one ketone to form the enolate quantitatively, then to react that enolate with the other carbonyl compound. Here, the use of LDA gives control of which compound forms the enolate, though it cannot be used to form aldehyde enolates.
Contributors
- Prof. Steven Farmer (Sonoma State University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Candela Citations
- Aldol reaction. Authored by: Martin A. Walker. Provided by: SUNY Potsdam. Project: Organic chemistry: An open textbook. License: CC BY-SA: Attribution-ShareAlike
- Aldol Condensation. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Organic_Reactions/Aldol_Condensation. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Synthesis of Enols and Enolates. Authored by: William Reusch and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Reactivity_of_Alpha_Hydrogens/Synthesis_of_Enols_and_Enolates. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Aldol Reaction. Authored by: William Reusch and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Reactivity_of_Alpha_Hydrogens/Aldol_Reaction. Project: Chemistry LibreText. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 23.5 Mixed Aldol Reactions. Authored by: Dr. Dietmar Kennepohl, Prof. Steven Farmer, William Reusch. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_23%3A_Carbonyl_Condensation_Reactions/23.05_Mixed_Aldol_Reactions. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 23.4 Using Aldol Reactions in Synthesis. Authored by: Dr. Dietmar Kennepohl and Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_23%3A_Carbonyl_Condensation_Reactions/23.04_Using_Aldol_Reactions_in_Synthesis. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Organic Chemistry With a Biological Emphasis. Authored by: Tim Soderberg. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/13%3A_Reactions_with_stabilized_carbanion_intermediates_I/13.3%3A_Aldol_reactions. Project: Chemistry Libretext. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike


