General mechanism for addition of weak nucleophiles to carbonyls
Weak nucleophiles are not strong enough to add to a simple C=O bond. They require the carbon to be made more electrophilic, and this can be done using either a Lewis acid or simply H+. We will focus on activation using simple acid – a process called protonation. This leads to an activated, charged form of the C=O which is now electrophilic enough to react with even weak nucleophiles via nucleophilic addition.
Most often the weak nucleophiles are uncharged compounds such as alcohols (ROH) or amines (RNH2 or similar). With these neutral nucleophiles, the product of the nucleophilic addition step is a positively charged compound, and so there is usually a final acid-base step to remove the unwanted H+ (“deprotonation”) and form the final uncharged product.
Mechanism
1) Protonation of the carbonyl
2) Nucleophilic addition to the activated carbonyl

3) Deprotonation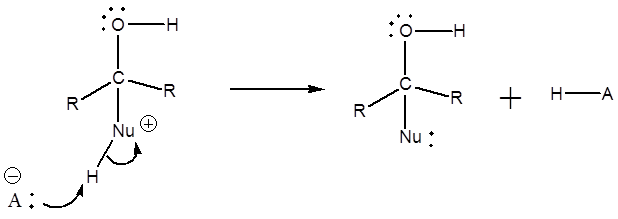
Contributors
- Prof. Steven Farmer (Sonoma State University)
Candela Citations
- General mechanism for addition of weak nucleophiles to carbonyls. Authored by: Martin A. Walker. Provided by: SUNY Potsdam. Project: Organic chemistry: An open textbook. License: CC BY-SA: Attribution-ShareAlike
- 21.7 General Reactions of Aldehydes and Ketones. Authored by: Prof. Steven Farmer . Located at: https://chem.libretexts.org/LibreTexts/University_of_Illinois%2C_Springfield/UIS%3A_CHE_269_-_Organic_Chemistry_II_(Morsch)/Chapters/Chapter_21%3A_Nucleophilic_Addition/21.07_Reactions_of_Aldehydes_and_Ketones%E2%80%94General_Considerations. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
